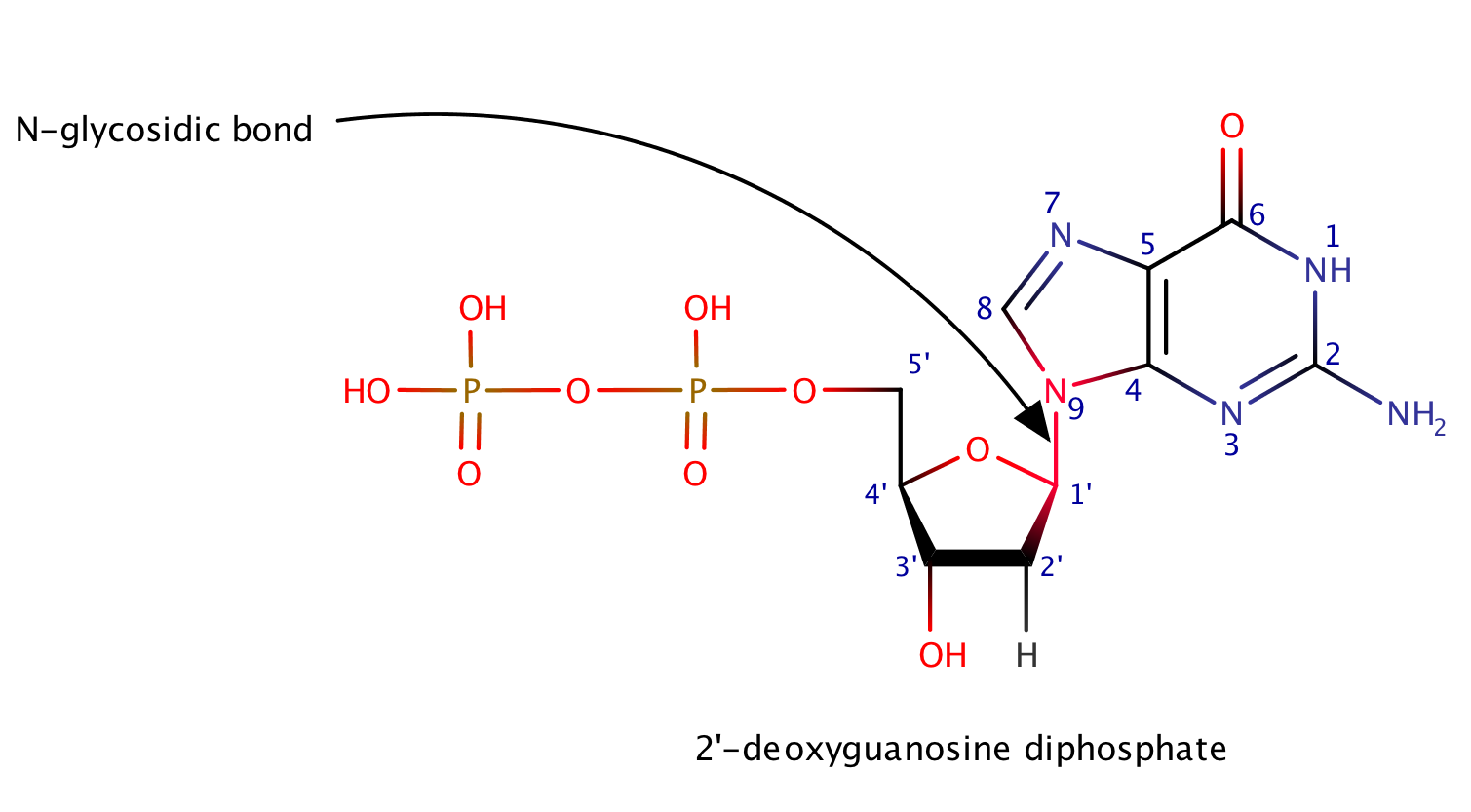
2'-deoxyguanosine diphosphate
2'-dGDP is a derivative of guanosine triphosphate (GTP), in which the -OH group on the 2' carbon on the nucleotide's pentose and one of the phosphoryl groups have been removed.[2] N-glycosidic bond[3] was marked with a sparrow and with a red color.
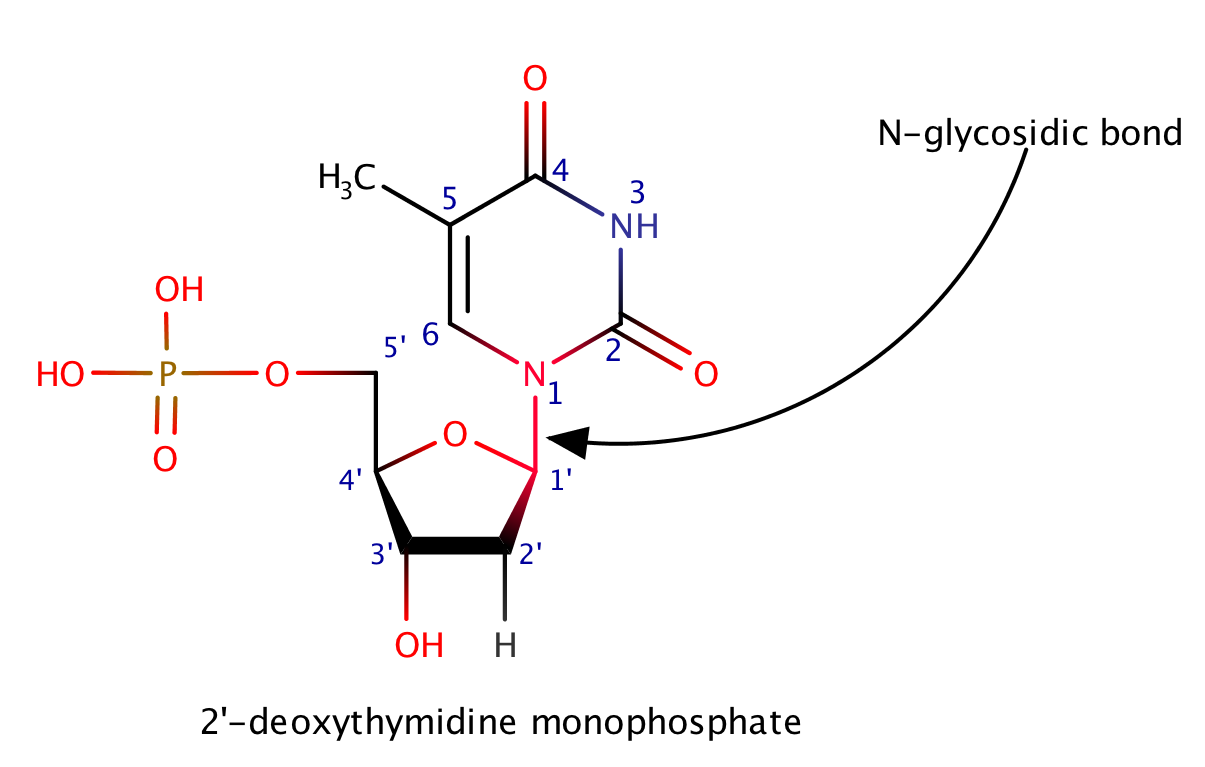
2'-deoxythymidine monophosphate
Also has the N-glycosidic bond; is a derivative pf thymidine triphosphate, in which the -OH group on the 2' carbon on the nucleotide's pentose and two of the phosphoryl groups have been removed.
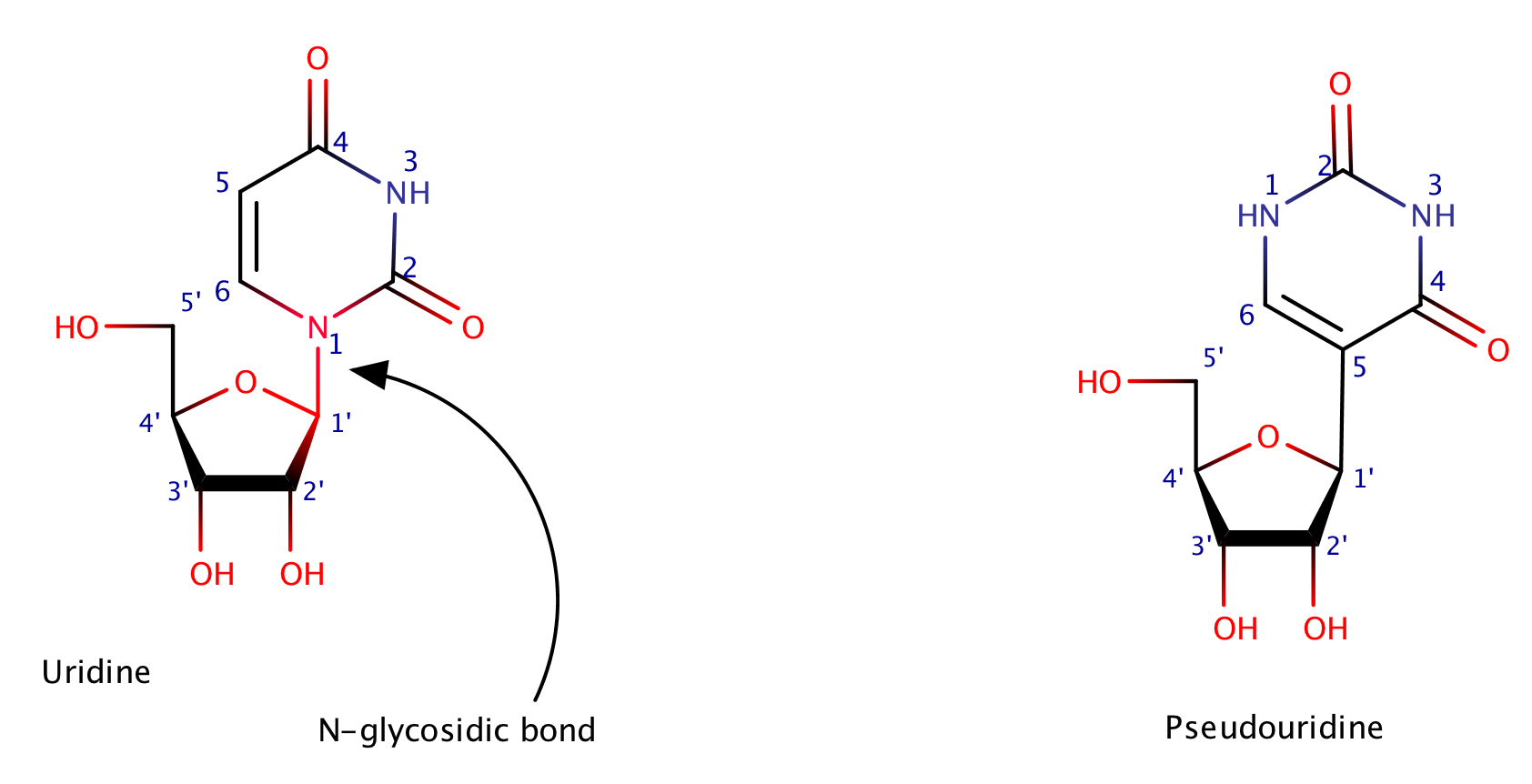
Uridine and pseudouridine
Unlike well-known uridine, pseudouridine doesn't have any N-glycosidic bond. It was the first modified ribonucleoside discovered. It can be found in structural RNAs (transfer, ribosomal and small nuclear RNA). Pseudouridine has been found to enhance base stacking and translation.[4]
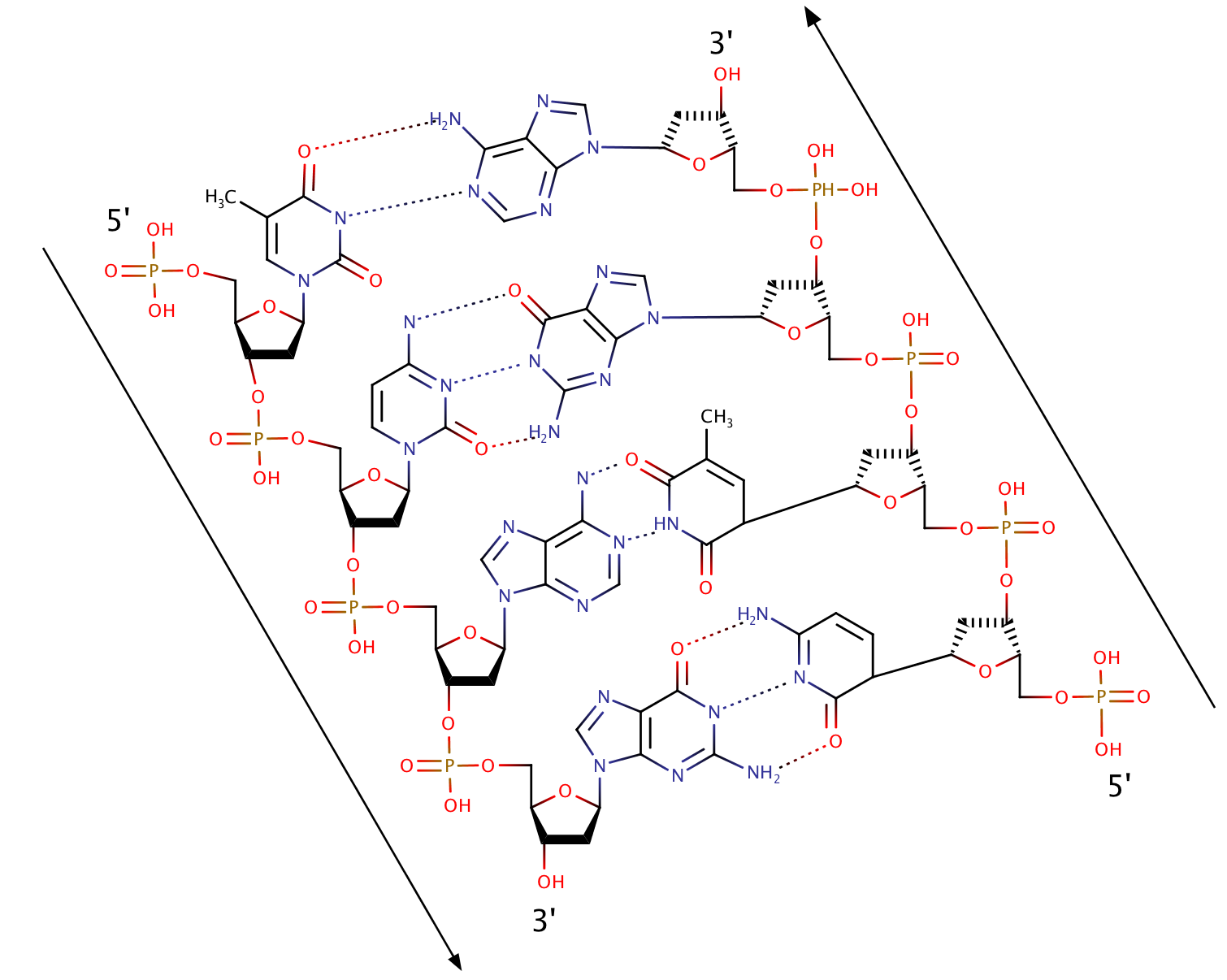
Part of a DNA structure
Well, I've tried to make it look like a picture from the presentation.