Atlas of interactions of acetone carboxylase purified from Xanthobacter autotrophicus
| RCSB-сode | 5M45 |
| Uniprot ID | ACXB_XANP2 |
| Classification | Ligase |
| Source file name | 5m45.pdb |
| Chains | 12 |
| Amino acid residues | 6544 |
| Ligands residues | 52 |
| Atoms | 51564 |
| Hetero atoms | 244 |
Introduction
Acetone carboxylases (ACs) catalyze the conversion of acetone and HCO3− to form acetoacetate. This process plays a key role in biological assimilation of toxic acetone molecules which are produced by the metabolism of certain anaerobic bacteria and from ketone body breakdown in mammals[1]. In general, bicarbonate-dependent carboxylases catalyze the dehydration of H2CO3 in two steps, retaining CO2 as a biotin adduct[2]. However ACs do not contain biotin so the mechanism of acetone transformation remained unknown. Situation changed after the announcement of structure of acetone carboxylase purified from Xanthobacter autotrophicus[3].
AC is a heteromultimeric complex with consists of αβγ subunits joined by the interacting α-subunits to form a dimeric core.
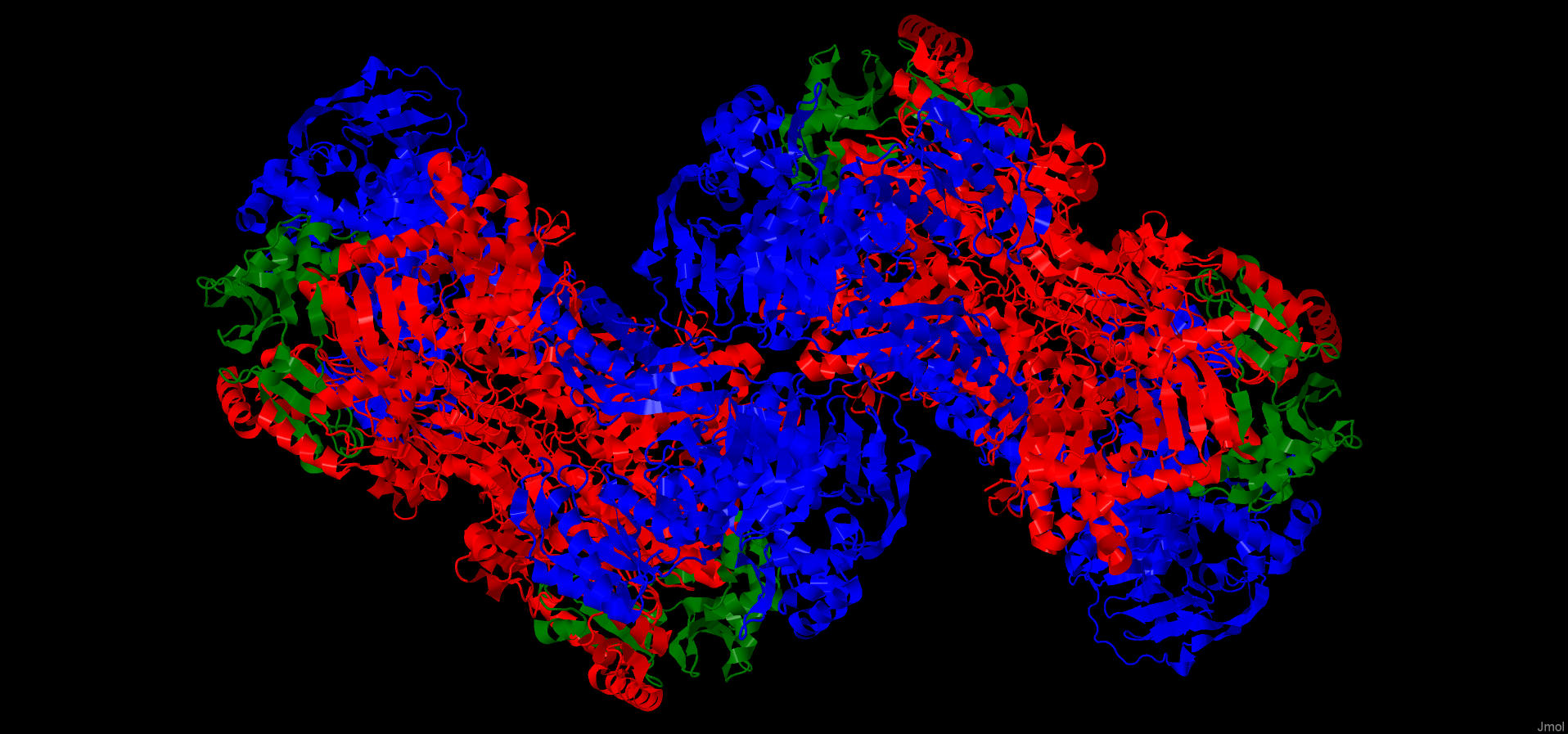
Subunits have different rates of conservation: α-subunit shows structural similarity to related acetophenone carboxylases (APCs). β-subunit shares nucleotide binding residues with homologous subunits of APC. γ-subunit in turn contains conserved cysteine residues that surrounds Zn2+ of active site (Fig. 7). Role of γ-subunit is not clear.
Structural peculiarity of AC is presence of eight PGII helices arranged into PGII sandwich-like structures[7]. These polyglycine type-II-like helixes have been discovered in synthetic polyglycines. Only four other structures within the PDB are known to contain six or eight PGII[8]. PGII helixes of AC α-subunit are proposed as an anchoring domain at the center of this multi-subunit complex[3].
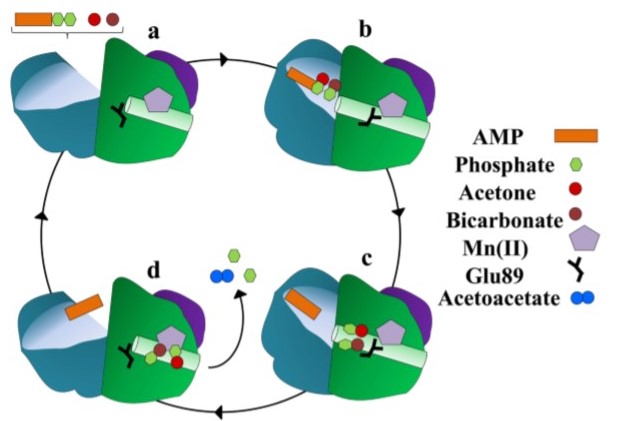
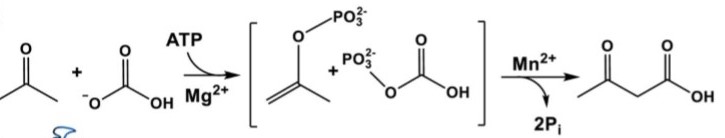
The figure above presents the main steps of ATP-dependent acetone carboxylation. Acetone and bicarbonate penetrate to the binding site through a substrate channel of β-subunit. Substrate binding triggers the closure of substrate channel and after that a new channel of α-subunit opens. This interior channel has AMP-bound part and connects the site at which acetone and HCO3- are phosphorylated with the Mn active site where acetone carboxylation occurs. The next stage of substrate channel cycle is that interior channel closes and the substrate channel reopens. After that acetoacetate may exit from the dimer interface[3].
Hydrogen bonds
Hydrogen bonds play an important role in protein secondary and tertiary structure formation. We observed this type of interactions using 'within' function of Jmol applet. Measurement of hydrogen bonds length was undertaken for 4_13 alpha-helices of different α-subunit chains and also for contact regions between α and γ and between α and β subunits. Hydrogen bonds between dimer subunits weren’t detected.
The hydrogen bond length within one subunit varies in the range of 3.1Å (for chains: A - 3,09; D - 3,07; G - 3,17; J - 3,07). In the case of two subunits interaction - in the range of 2,93Å. These values are close to that described in literature[9].
First applet
Ligands
| IUPAC | Magnesium (2+) |
| Brutto-formula | Mg2+ |
| PubChem ID | 888 |
| Molar mass | 24,3 g/mol |
| IUPAC | Manganese (2+) |
| Brutto-formula | Mn2+ |
| PubChem ID | 27854 |
| Molar mass | 54.938 g/mol |
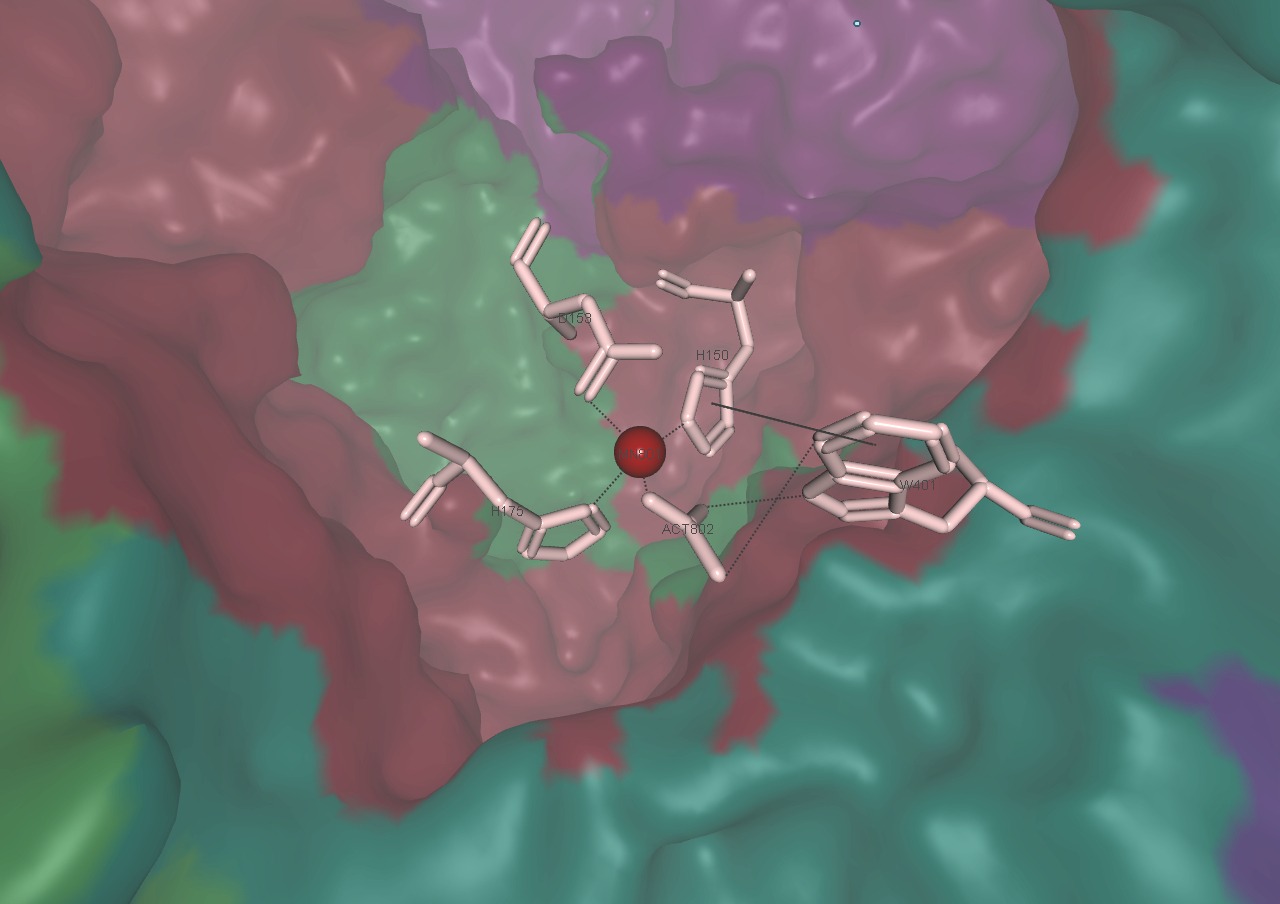
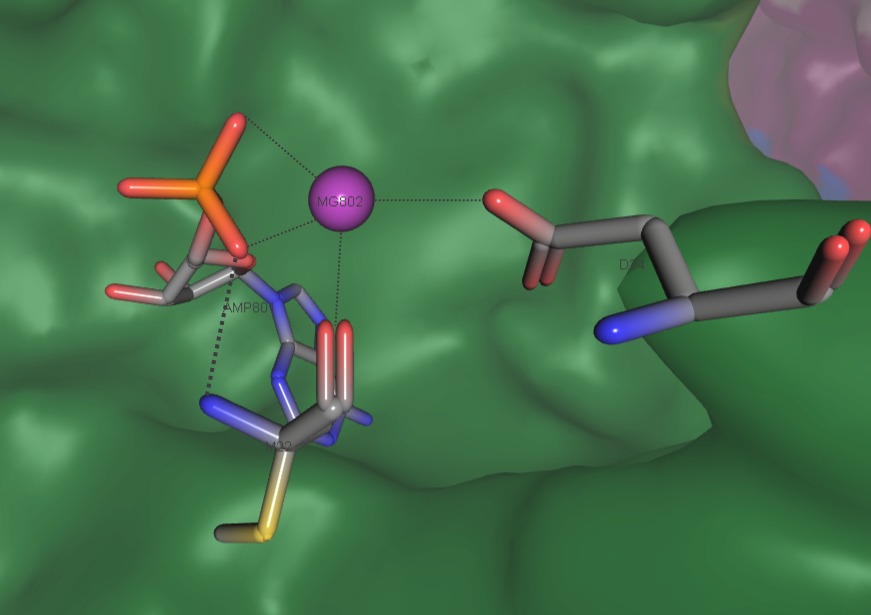
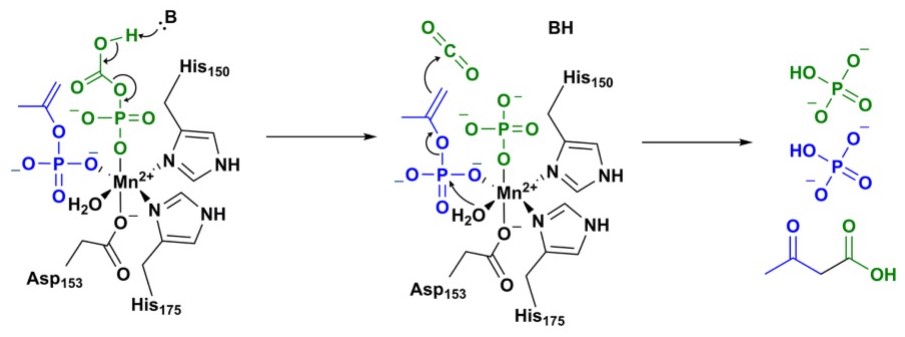
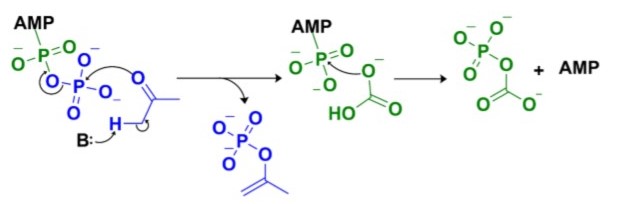
Intermediates phosphoenolacetone and carboxyphosphate are proposed to react together at the Mn2+ active site of α-subunit to create acetoacetate and two molecules of inorganic phosphate (Fig. 6)[3].
| IUPAC | Zinc ion |
| Brutto-formula | Zn2+ |
| PubChem ID | 32051 |
| Molar mass | 65.38 g/mol |
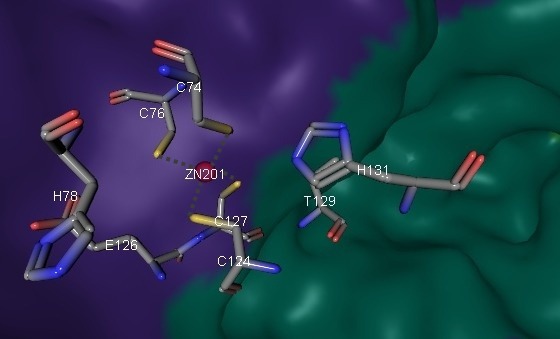
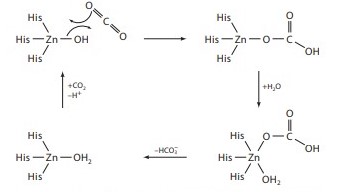
pKa of zink ion is not higher than physiological pH value so it can rapidly activate a water molecule and form [Zn-OH]+. Possible mechanism of CO2 capture involving Zn2+ is presented on Fig. 8. The role of the AC γ-subunit is not clear, and the zink ion is not predicted, from prior data, to have a catalytic role.
| IUPAC | [(2R,3S,4R,5R)-5-(6-Amino -9H-purin-9-yl)-3,4-dihyd roxytetrahydro-2-furanyl] methyl dihydrogen phosphate |
| Brutto-formula | C10H14N5O7P |
| PubChem ID | 6083 |
| Molar mass | 347.224 g/mol |
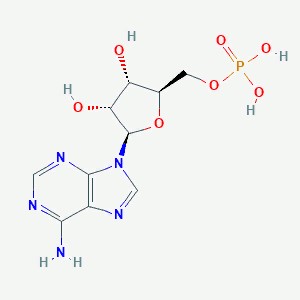
The process of AMP formation could be described by activation of acetone by ATP to generate a phosphoenolacetone intermediate and ADP that then reacts with bicarbonate. That results in generation of carboxyphosphate and AMP. AMP serves as linker of a current protein state: the AMP-bound structure exhibits dramatic rearrangements of the β-subunits relative to the ligand-free state (Fig. 10).
| IUPAC | Acetate ion |
| Brutto-formula | C2H3O2 |
| PubChem ID | 175 |
| Molar mass | 59.04 g/mol |
| IUPAC | 2-[2-[2-[2-(2-ethoxyethoxy)ethoxy] ethoxy]ethoxy]ethanol |
| Brutto-formula | C12H26O6 |
| PubChem ID | 78058 |
| Molar mass | 266.33 g/mol |
It is the target of mitochondrial Malonyl-CoA-acyl carrier protein transacylase which catalyzes the transfer of a malonyl moiety from malonyl-CoA to the free thiol group of the phosphopantetheine arm of the mitochondrial ACP protein[6]. In acetone carboxylase function is unknown.
Second applet
There were no disulfide bridges found within the protein (verification was performed using command 'restrict cys').
Hydrophobic interactions
Because no valuable information about intra-protein interaction was found in articles and the computer capabilities of the cite conducting similar calculations (http://pic.mbu.iisc.ernet.in/job.html) were not sufficient for the goals of the research, all following examples were found and calculated manually. Tryptophan-438 of α-subunit and its surrounding are shown as an example of hydrophobic interaction. It begins to act beyond 3Å and at the distance more than 5Å the ambience of the central residue is so dense that it cannot be seen behind its neighbors. According to measurements, an average distance between adjacent not bound covalently atoms is about 3.54Å. Van der Waals radiuses of atoms in residues are higher than 1.4Å, hence, the distance between them is less than 0.74Å. Water molecule (or any atom) can not fill this gap as electrostatic force would push it out.
Salt bridges
The bond between arginine (339) and glutamic acid (746) residues in α-subunit is represented as an example. Characteristics of the interaction are shown using the command ‘measure’ and match the average distance for ionic bonding.
Stacking
T-shaped stacking is found between two phenylalanine’s residues (334 and 352) in α-subunit. According to the measurements shown in the following applet, the relevant distance between aromatic rings is about 3.77Å.
Third applet
Author contributions
Daria Nogina chose the protein, wrote the review of its properties and functions, analyzed hydrogen bonds and ligands. Angelika Dodonova unsuccessfully tried to find disulfide bridges, described stacking, salt bridge and hydrophobic interaction, designed the html-page and also translated the project into russian.
References
[1] http://www.jbc.org/content/279/45/46644.short
[2] Tong, L. Structure and function of biotin-dependent carboxylases. Cell Mol. Life Sci. 70, 863–891 (2013)
[3] Mus, Florence Structural Basis for the Mechanism of ATP-Dependent Acetone Carboxylation. Scientific Reports, 7234, 7, 1 (2017)
[4] Chovancova, E. et al. CAVER 3.0: a tool for the analysis of transport pathways in dynamic protein structures. PLoS Comput. Biol. 8 (2012)
[5] Ivano Bertini Biological Inorganic Chemistry Structure and Reactivity. UNIVERSITY SCIENCE BOOKS Sausalito, California
[6] DrugBank http://www.drugbank.ca/drugs/DB07344#targets
[7] https://www.sciencedirect.com/science/article/pii/S0969212618302636
[8] https://www.rcsb.org
[9] Arunan, E., Desiraju, G., Klein, R., et al. (2011). Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure and Applied Chemistry, 83(8), pp. 1637-1641. Retrieved 4 Mar. 2019, from doi:10.1351/PAC-REC-10-01-02
[10] https://onlinelibrary.wiley.com/doi/full/10.1002/chem.200800987