|
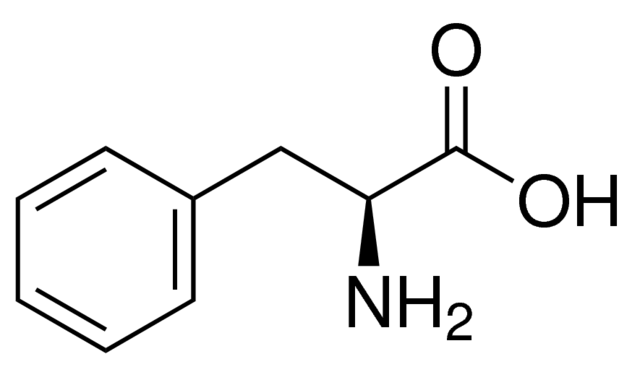
The right figure shows a two-dimensional structure of L-phenylalanine, which refers to an aromatic α-amino acids [2]. As you can see, this amino acid has the radical
-CH 2-C 6H 5. Due to the fact that the radical is a benzyl group which exhibits hydrophobic and inert properties, the amino acid is
neutral molecule. Phenylalanine is the precursor of a variety of molecules, such as tyrosine, norepinephrine, epinephrine ... Phenylalanine is an essential amino acid, so
it is not synthesized de novo in human bodiy. As for the synthesis of phenylalanine, GMO bacteria E. coli are very popular commercial bacteria of phenylalanine synthesizing [5].
|
Titles sequence.
| Table 1. Properties of L-phenylalanine [3] |
| Physical and chemical properties | | Common info | |
| Density | 0.754 g/cm3 | Russian title | Ôåíèëàëàíèí |
| Chemical formula | C6H5-CH2-ÑH(NÍ2)-ÑÎÎÍ | Three-letter code | Phe |
| Brutto-formula | C9H11NO2 | One-letter code | A |
| Molar weight | 165.18914 g/mole | RNA coding codons | UUU, UUC |
| Solubility in water | 26.9 g/l | IUPAC title | 2-amino-3-phenylpropanoic acid |
| Standart state | White cristallic | PubCHEM ID | CID 6140 |
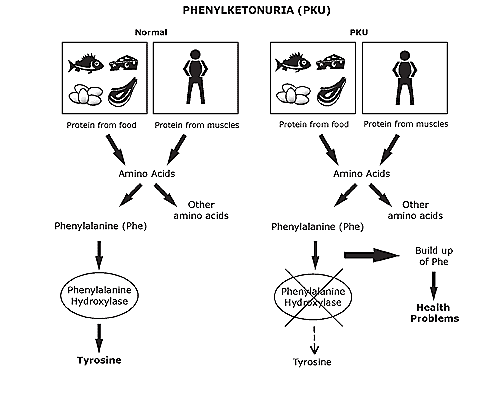 |
| Fig. 1. Phenylketonuria scheme (Source).
|
On the right side you can see a figure of phenylketonuria (PKU) scheme
[1]. It is an innate disorder that occurs with the full or partial absence of the enzyme, phenylalanine hydroxylase, activity.
Thus, food rich in protein is dangerous for people suffering from PKU.
|
Properties of L-phenylalanine pH
Protonated form of amino acid observed in the value of pH < 1.83, while there is a charge on NH3+-group, COOH-group remains uncharged. L-phenylalanine becomes a zwitterion
(charged NH3+ and COO- groups) at pH = 1.83. Only COO--group (without NH3+-group) charges starting with values of pH = 9.13.
|
pKa 1.83 sequence.
pKa 9.13 sequence.
pH < 1.83 sequence.
|
| Table 2. Protein-protein interaction |
| Hydrogen bonds | Peptide binding/gydrophobic interaction |
| Passing through the bone H | Passing through the bone O | Bone/bone |
|
|
|
|
|
|
|---|
| Scriptmegafile! |
|---|
|
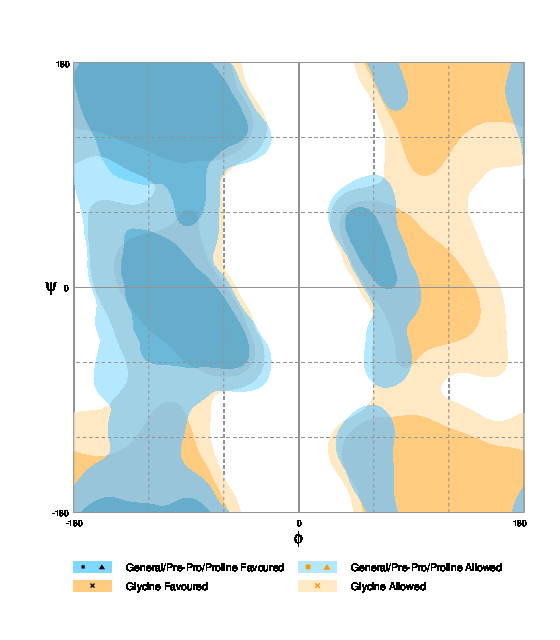 |
| Fig. 2. Phenylalanine Ramachandran plot (Source).
|
Protein-protein interaction is a specific physical interaction between two or more proteins that occurs as a result of
biochemical reactions and/or electrostatic interactions. Notable examples of protein-protein interactions are homo-oligo(poly)meres/hetero-oligo(poly)meres,
stable complexes, covalent/non-covalent complexes.
Factors that regulate protein-protein interactions:
- Protein concentration
- Protein affinity
- Ligand concentration
- Electric fields around proteins
- Presence of nucleic acids, other proteins, ions...
Phenylalanine radical is not capable of hydrogen, ionic bonds. However, it is capable of participating in the formation of hydrophobic bonds, due to the absence strong electronegative atoms in the structure of the radical.
It is also possible to note the presence of the benzyl group of radical. Thus it can be said that phenylalanine refers to aromatic amino acids.
Interactions of DNA molecule and alanine radical were not detect. This is also confirmed by the theory. Alanine radical is hydrophobic, nonpolar, that means
that alanine can not interact with the charged DNA molecule.
Information sources:
[1]: Toxnet
(Source)
[2]: Wikipedia
(Source)
[3]: PubChem
(Source)
[4]: RCSB
(Source)
[5]: NCBI
(Source)