|
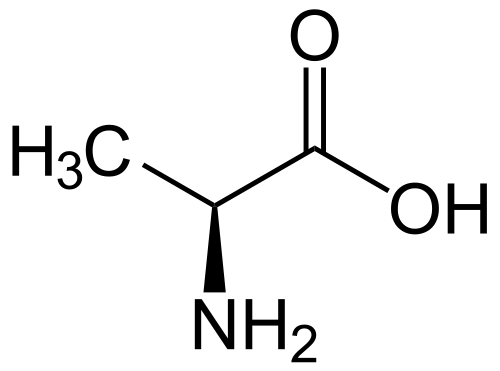
The figure on the right side shows a two-dimensional structure of L-alanine, which refers to an aliphatic α-amino acids [2]. As you can see, this amino acid has the radical
-CH 3-group, a methyl. Because of its simplicity relative to other amino acid alanine is unreactive, therefore almost not involved
in the functioning of the protein. Alanine is a nonessential amino acid, so the body does not necessarily receive this amino acid constantly. There are several ways of
synthesis, such as the biosynthesis and chemical synthesis in the laboratory. In vivo alanine often synthesized from pyruvate and amino acids with branched radicals, for instance
valine, leucine, isoleucine [5].
|
Titles sequence.
| Table 1. Some properties of L-alanine [3] |
| Psychical and chemical properties | | Common info | |
| Density | 1.424 g/cm3 | Russian title | Àëàíèí |
| Chemical formula | NH2-ÑH(ÑÍ3)-ÑÎÎÍ | Three-letter code | Ala |
| Brutto-formula | C3H7NO2 | One-letter code | A |
| Molar weight | 89.09 g/mole | RNA coding codons | GCU,GCC,GCA,GCG |
| Solubility in water | 167.2 g/l | IUPAC title | 2-aminopropanoic acid |
| Standart state | White criastallic | PubChem ID | CID 5950 |
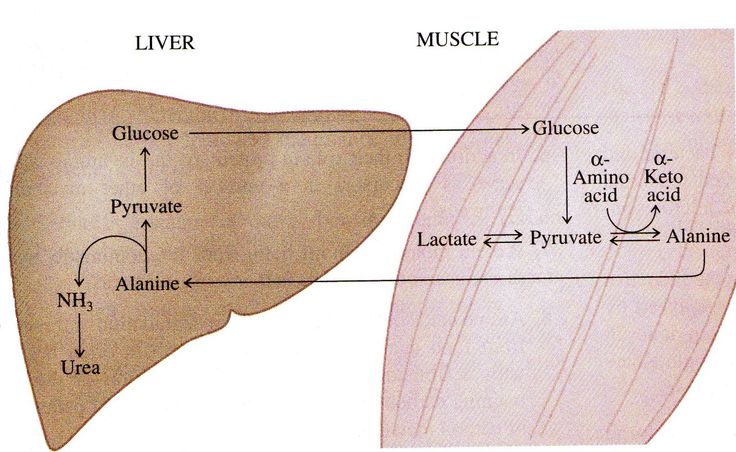 |
| Fig. 1. Glucose-alanine cycle (Source).
|
At the right side you can a figure of the glucose-alanine cycle
[1]. In the first step ïlucose converted to pyruvate (glycolysis),
which is converted to alanine, by the action of alanine aminotransferase. Further, alanine is carried by the blood to the liver where the enzyme having the opposite effect
to muscle aminotransferase converts alanine to pyruvate. Further, pyruvate converts to alanine by gluconeogenesis. The cycle is repeated.
This cycle is one of the most important in the human body, as it allows the muscles to constantly obtain glucose, which is the most important energy product of muscles.
|
Properties of L-alanine pH.
Protonated form of amino acid observed in the value of pH < 1.83, while there is a charge on NH3+-group, COOH-group remains uncharged. L-alanine becomes a zwitterion
(charged NH3+ and COO- groups) at pH = 1.83. Only COO--group (without NH3+-group) charges starting with values of pH = 9.13.
|
pKa 2.34 sequence.
pKa 9.69 sequence.
pH < 2.34 sequence.
|
| Table 2. Protein-protein interaction |
| Hydrogen bonds | Peptide binding/gydrophobic interaction |
| Passing through the bone H | Passing through the bone O | Bone/bone |
|
|
|
|
|
|
|---|
| Scriptmegafile! |
|---|
|
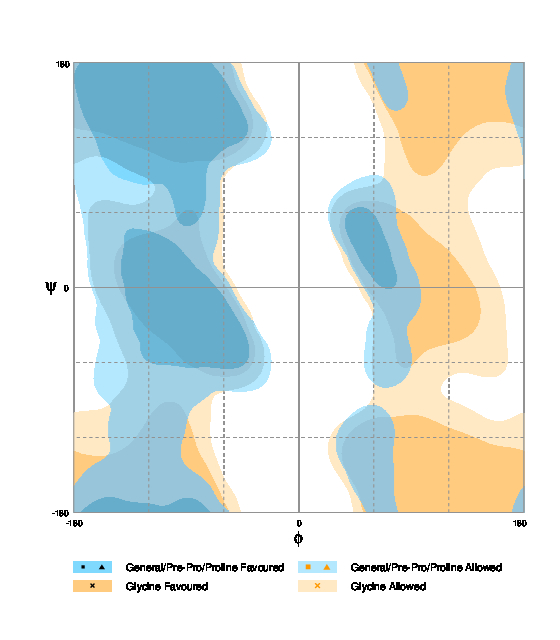 |
| Fig. 2. Alanine Ramachandran plot (Source).
|
Protein-protein interaction is a specific physical interaction between two or more proteins that occurs as a result of
biochemical reactions and/or electrostatic interactions. Notable examples of protein-protein interactions are homo-oligo(poly)meres/hetero-oligo(poly)meres,
stable complexes, covalent/non-covalent complexes.
Factors that regulate protein-protein interactions:
- Protein concentration
- Protein affinity
- Ligand concentration
- Electric fields around proteins
- Presence of nucleic acids, other proteins, ions...
Alanine radical is not capable of hydrogen, ionic bonds. However, it is capable of participating in the formation of hydrophobic bonds, due to the absence strong electronegative atoms in the structure of the radical.
Interactions of DNA molecule and alanine radical were not detect. This is also confirmed by the theory. Alanine radical is hydrophobic, nonpolar, that means
that alanine can not interact with the charged DNA molecule.
Information sources:
[1]: Toxnet
(Source)
[2]: Wikipedia
(Source)
[3]: PubChem
(Source)
[4]: RCSB
(Source)
[5]: NCBI
(Source)